Founded in 2007, Biophore India Pharmaceuticals Pvt. Ltd has established itself as a trusted partner for niche and complex products. With 4 US FDA and EU approved API manufacturing facilities, one dedicated intermediate facility and a world class R&D lab housing 400 scientists with varied expertise, Biophore has emerged as one of the leading API companies globally. We have consistently been in the Top 10 US DMF filers with the US FDA over the past 5 years and with most of our APIs, we are one of the fastest companies to bring them to the market enabling wider access for patients worldwide.
Biophore Released Job Openings
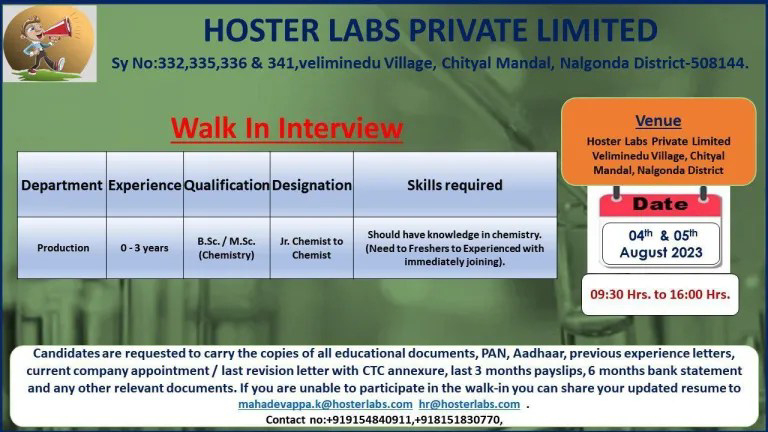
Qualification: M.Pharm, M.Sc
Job Location: Hyderabad/ Secunderabad,Telangana, Bangalore/Bengaluru
Department: Regulatory affairs -API
Experience : 03- 06+Years
Role: Executive/Sr.Executive
Salary: 2-6 Lacs P.A.
Job description
Roles and Responsibilities
DMF submissions, Annual Reports, Variations, Renewals etc. for US and Europe market.
Compilation and publishing in eCTD/CTD format for Submission to health agencies like EDQM, USFDA etc.
Oversee final technical quality review and technical validation (PDF/eCTD/NeeS)
Review all regulatory agency submission materials for accuracy, comprehensiveness, or compliance with regulatory standards.
Provide deficiencies responses to HA regulatory agencies for deficiencies and comments
Evaluation of change controls for post-approval changes (PAC)
Compilation, review and submission of Variations
Keeping up to date with changes in regulatory legislation and guidelines
Conduct Pre-submission and Initial Submissions meetings
Preparation of gap analysis report of CMC regulatory submission including recommendations to facilitate the review of application by HA
Gaps in manufacturing and controls, assessment of regulatory risk and strategy
Providing gap analysis against applicable HA requirements and standards and communicate cross functional team to comply the samezCharacterization of the API and Raw materials used to manufacture the API
Description of the product and process development
Analytical methods and specifications used for testing
Release and stability testing data for both the API
Preparation of responses to HA,comment letters&assessment reports
Initial assessment of the submission (initial / post approval submissions) E.g., initial validation comments, response to the completeness assessment report, response to the Information Requests









.jpg)